Photo electric effect is where we study behavior of metals when electromagnetic radiations falls on them. When metals are provided with some energy, electrons in their atoms are extracted and comes to the surface. This energy that extracts electrons from metal surface will come from heat energy or from electromagnetic radiations.
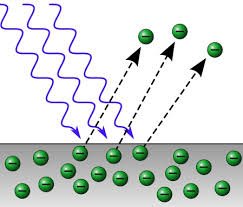
When Electromagnetic waves are incident onto a metal surface, some electrons gains enough energy to escape from the surface. For instance, when light energy falls on a negatively charged conductor, it becomes discharged.
The emission of electrons by a metal exposed to light is known as the photoelectric effect.
Electrons emitted by photoelectric effect are referred to as photoelectrons.
A material that exhibits photoelectric effects is said to be photoemmisive.
Illustrating Photoelectric effects
To experiment on photo electric effects, you may need the following materials.

An electroscope

zinc plate

Emery paper

glass rod

silk cloth

Ebonite rod

piece of fur

Table lamp

Ultraviolet lamp

sheet of glass
proceed as the follow:
- Clean the surface of the zinc plate with emery paper and lay it on the electroscope cap.
- Rub the glass rod with silk and charge the electroscope positively.
- Shine light from the table lamp onto the zinc plate and observe if the electroscope leaf falls or rises
- Repeat the steps above using ultraviolet lamp and observe if the electroscope leaf falls or rises
- Discharge the electroscope and lay the zinc plate on it again
- Rub the ebonite rod with fur and then charge the electroscope negatively
- Shine light from the table lamp onto the zinc plate making the light as intense as possible by holding it as close as possible to the surface and see if the electroscope leaf falls or rises
- Repeat 6 and 7 using ultraviolet lamp and observe if the electroscope leaf falls or rises.
- Recharge the electroscope negatively and try to discharge it by shining ultraviolet light on the zinc plate as shown
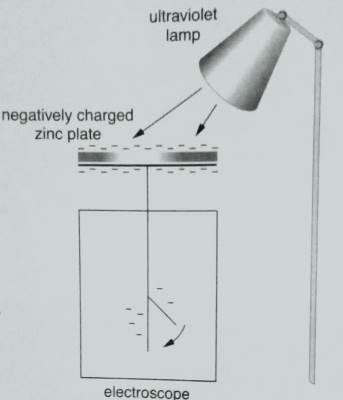
As the leaf begins to fall, insert a sheet of glass between the lamp and the plate.
Observations on Photoelectric effect experiment
- When visible light from table lamp was shone onto the positively charged electroscope, the leaf did not fall.
- when ultraviolet rays from ultra violet lamp are directed on to the positively charged electroscope, the leaf did not fall.
- When visible light was shone onto the negatively charged electroscope, the leaf of the electroscope did not fall.
- When ultraviolet rays were directed towards the negatively charged electroscope, the leaf of the electroscope falls.
- When a sheet of glass is placed between the violet lamp and the discharging electroscope, it stops the discharge. Discharging continues when it is removed.
Explaining the experiments on photoelectric effects
Neither visible light nor ultraviolet rays were able to discharge positively charged electroscope. This is because there are no electrons to be dislodged from positively charged electroscope. This suggests that the effects of radiations is to eject electrons which has negative charges from the metal surface.
The fact that visible light is not able to discharge a charged electroscope while the ultraviolet rays does suggests that not all the radiations can eject electrons from a metal surface. Ultra violet rays has a higher frequency compared to visible light suggesting that a certain minimum energy is required to eject electrons from a metal surface.
A sheet of glass stopping the discharging process suggests that the glass block absorbs the ultraviolet rays and so they are not able to penetrate through and reach the zinc plate.
One reason a positively charged electroscope is not discharged by radiations is that photoelectrons emitted from the positively charged zinc plate do not escape as they are neutralized by the positive charges on the plate and so the leaf divergence remains the same.
Illustrating photoelectric effect using a photocell
A photocell contains two metal electrodes sealed in an evacuated tube. The figure below shows a photocell circuit.
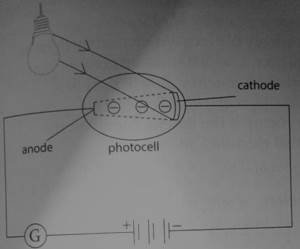
Evacuation of the tube prevents electrons from being stopped by air molecules.
The tube is usually made of quartz material in order to allow ultraviolet rays pass through easily. Electrons after being ejected at the cathode, they are attracted to the anode by a potential difference across the electrodes.
Before any radiation falls on the cathode, no deflection is noted on the galvanometer. This means that no current is flowing through the circuit. When a radiation of given frequency falls on the cathode, deflection is observed on the galvanometer meaning that current has been flowed through the circuit.
Electrons after being ejected from the cathode, are accelerated towards the anode by the potential difference hence the circuit is complete.

Leave a Reply