The term “properties of cathode rays” refers to the various physical characteristics and behaviors observed when cathode rays are studied under different conditions.
properties of cathode rays includes:
- They travel in a straight line
- They cause fluoresce or glow to certain materials
- they are charged
- They possesses kinetic energy
- pass through thin materials, demonstrating their ability to penetrate objects to varying degrees depending on the material’s density and the energy of the rays.
- ionize gases
- The charge-to-mass ratio of cathode rays (electrons) is relatively high. A property used to identify the electron and distinguish it from other particles.
- Deflection by Magnetic Fields
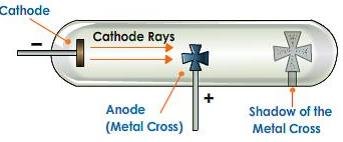
Showing that cathode rays travels in a straight line
When an opaque object is placed between the screen and the cathode in the path of the cathode rays, a sharp shadow is cast on the screen.
Cathode rays causes certain substances to glow or fluoresce
Fluoresce materials are materials that glows when electromagnetic energies falls on them. such materials includes zinc sulphide, Fluorescein, Rhodamine, Coumarin, Acridine Orange and Quantum Dots.
when cathode rays falls on screen coated with the fluoresce materials, the fluoresce material glows.
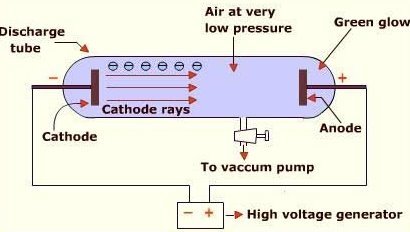
showing that cathode rays are charged
Cathode rays are deflected by both magnetic and electric fields.
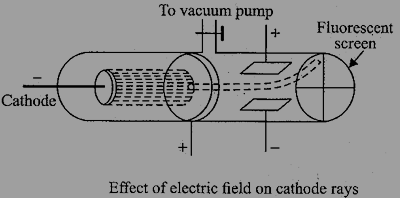
Inside the magnetic field, the cathode rays are deflected towards the positive plate showing that they are negatively charged. Remember that opposite charges attract while while same charges repel from the basic law of charges. see the figure below:
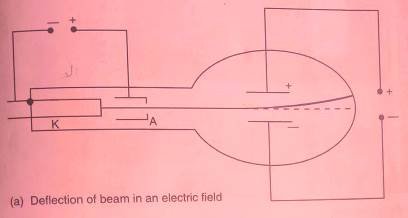
When cathode rays passes through magnetic field, they are deflected in the direction determined by Fleming’s left-hand rule. The deflection in magnetic field shows that they are negatively charged as shown in figure below.
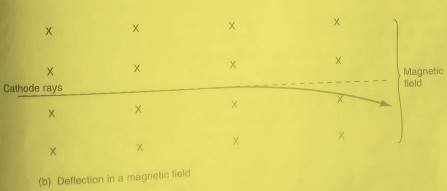
Cathode rays have kinetic energy
The deflection of cathode rays in a magnetic field shows they are moving, and therefore possess kinetic energy. By measuring how much the cathode rays bend in the magnetic field, you can calculate their velocity. Using the velocity, you can compute the kinetic energy of the cathode rays.
When cathode rays are suddenly stopped by a metal target, they can produce x-rays. This confirms that they are actually a stream of fast moving electrons.
Exam Questions on properties of cathode rays
Figure 14 shows a cathode ray tube. A metal plate is placed between the anode and the screen.

(I) State with reason what would be observed on the screen when the cathode rays are produced. (2 marks)
(ii) State the effects on the cathode rays produced when the anode is increased. ( 2 marks)

Leave a Reply